In collaboration with the working group of Prof. Al-Shamery (Physical Chemistry, University of Oldenburg) we develop precursors which can be decomposed yielding ultrathin oxide layers. Oxides with high dielectric constants are of special interest ("high-k-materials"), which play a crucial role in the process of miniaturization of electronic components. Suitable oxides include either rare earth elements, early transition metals, or refractory metals. A fundamental feature of our deposition process is the application of carbon free precursors or compounds, whose thermal decomposition is stamped by inner redox reactions which lead to the oxidation of the carbon containing parts of the structure. This is achieved in both cases with the help of nitrates, which are available in stabilized molecular or in an ionic form. The latter are nitronium or nitrosylium salts, which can be synthesized very neat with N2O5. Initially the molecular and ionic nitrates of the rare earth elements are synthesized and comprehensively characterized. In all cases it will be investigated how the composition and the chemical nature of the ligands applied influence the structure and contamination of the prepared oxide layers. The characterization is achieved with spectroscopic (XPS) and electron microscopic methods. In cooperation with the working group of Prof. Lee (School of Material Science and Engineering, University of Singapore) the electronic characteristics of the layers are determined. A second but also important feature of our application is the deposition with easy, efficient, and cheap methods like dip or spray coating. The use of these applications for the coating of silicon surfaces has already been patented successfully. These procedures may also be of further interest for the coating of labile substrates with oxides.
Fig 1: Nd2O3 layer on a Silicon (111)-Surface (left). For the measurement of the electronic properties (right) the Nd2O3 layer was coated with a layer of TiO2 and gold electrodes.
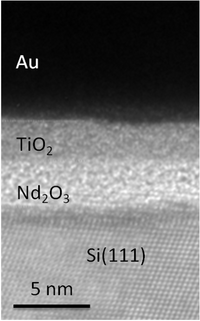
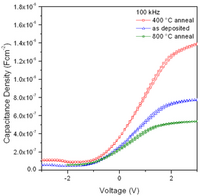
Abb 1: Nd2O3-Schicht auf einer Silicium (111)-Oberfläche (links). Zur Messung der elektronischen Eigenschaften (rechts) wurde die Nd2O3-Schicht mit einer Schicht TiO2 und Goldelektroden belegt.